Our discussion today is something that most persons might not really bother to think about, especially if you are not science inclined. For us as researchers and for me in particular, virtually everything that seems weird arouses my curiosity. As they would always say, curiosity breeds innovation.
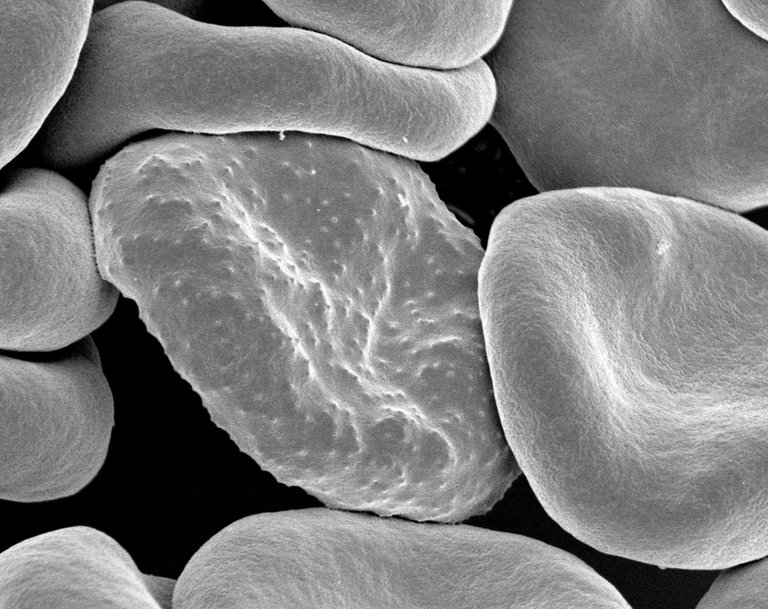
Human red blood cells
I was in a deep thought about our human blood cells and how they continuously circulate within the body's circulatory system without forming a flocculate (aggregating together). What really causes and prevents this from happening and if it does happen, why? Imagine the millions of blood cells freely circulating inside the body without having to aggregate in normal situation, indeed it is worth reasoning!
The above births our discussion for today. Reading this article, you will get a deeper insight on what happens and how the blood is able to do this without any challenge. Join me as I unravel the mystery behind the non flocculating nature of the human blood cells. As usual, let's look at the structure of the human blood and vascular system. This will give us a better landing spot.
The human circulatory and vascular system
The human blood red cells are biconcave in shape with a pale central pallor for adequate oxygen transportation to various organs and tissues. Some disease conditions like sickle cell can cause the blood to be inefficient in carrying out this one important function. Sickle cell disease causes the blood to loose it's normal biconcave shape as well as its able to maneuver and pass through the blood vessels.
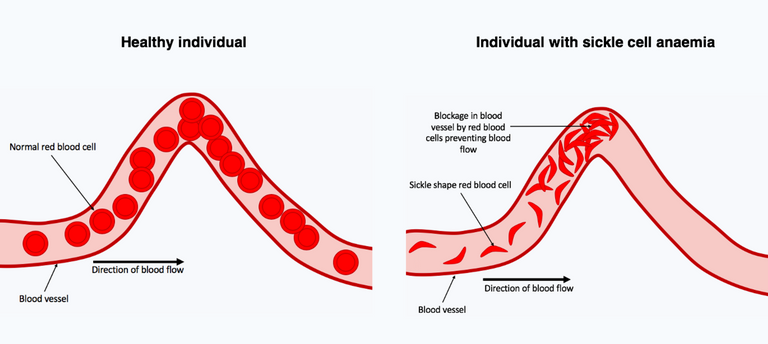
Sickle cell red blood cells in the blood vessels
All these can culminate to what is known as hypercoagulability of the blood (i.e. tendency of the blood to clump together and form coagulation or thrombos depending on where it occurs). All things being equal, the blood is not by any means expected to clot within the blood vessels unless in cases of endothelial injury and some tissue repair processes.
The three factors namely - Hypercoagulability, Venous stasis, and Endothelial injury/dysfunction individually initiate and contribute to the formation of thrombosis. Absence of any this factor means that the blood cannot clot nor form flocculates. But there is more to this that some of us don't know, read on, you you will find out.
As we earlier explained, hypercoagulability is simply the increased tendency of the blood cells to form coagulation or clump together easily instead of moving and flowing freely within the blood vessels. Aging red blood cells are more prone to hyper-coagulation, hence, they are destroyed by one of the reticuloendothelial system - the spleen.
Cells with high tendency of always clumping together can cause the formation of thrombus. Furthermore, another factor put into consideration is referred to as Venous Stasis. This simply refers to those haemodynamic changes that affects the movement of the blood cells within the vessels.
Naturally, blood cells flow in a laminar manner without any form of obstruction, but in situation where there is turbulent or haphazard flow, the tendency of the blood to clump is extremely high thus, leading to thrombus formation. It becomes worst if the blood cells are not constantly flowing.
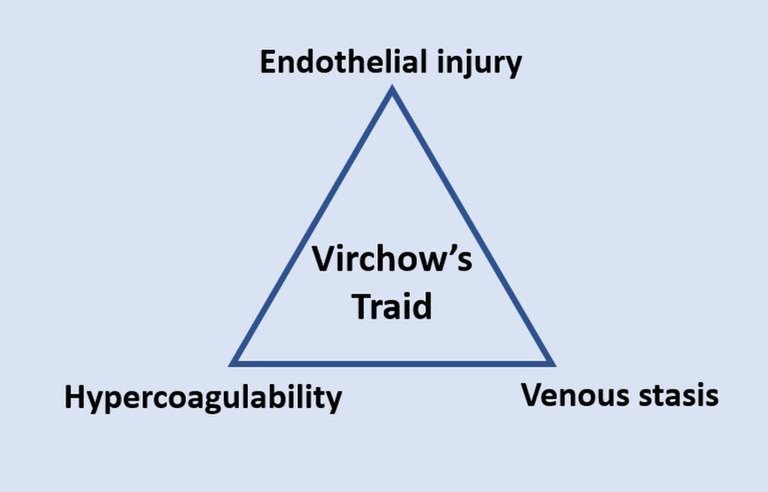
The Virchow's triad
The third factor which is endothelial injury or dysfunction refers to injury to the smooth endothelial wall of the blood vessels. Should anything happen to the endothelial wall, the consequence is a activation of the clotting system which comes in to repair the damage. These are the major factors or conditions that when disrupted or activated could trigger clumping of the blood cells. The three conditions described above forms what is known as the Virchow's triad.
By nature, the endothelial wall is smooth and this is ensured by vasoactive factors like Nitric oxide (NO), Prostacyclin (PGI2), TXA2 etc. All this factors work to maintain the antithrombogenic (inability to form clot) nature of the blood vessels. For detailed read, you can read this post from which some of the excerpts above were extracted from - In-depth expository into Coronavirus Vaccine-Induced Thrombotic thrombocytopenia and associated side effects.
Imagine that the wall or route through which blood moves is sticky or adhesive in nature, it simply means that the cells will stick to the walls and should this continue, there will definitely be clumping and serious aggregation of cells thus leading to blood flow obstruction.
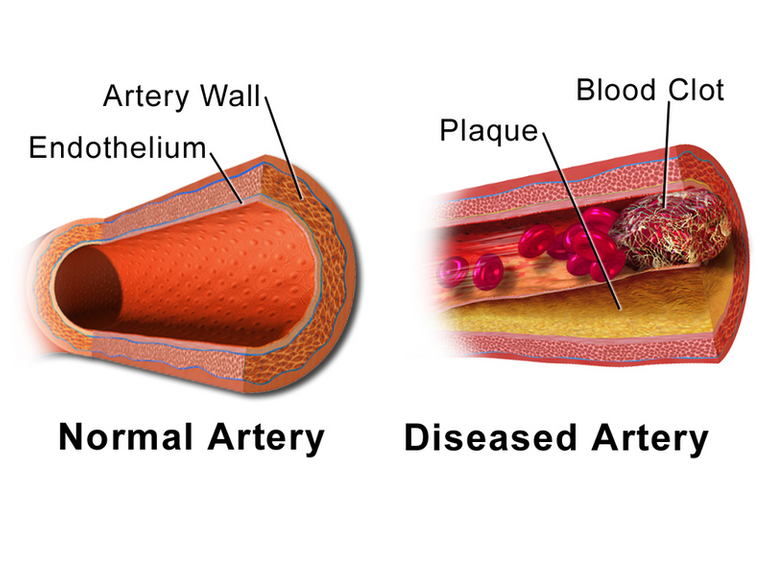
Structure of the blood vessels
The smoothness of the endothelial walls ensure the easy movement and flow of blood, but what if the blood cells themselves are sticky in nature; will the smoothness of the endothelial wall be enough to allow them still flow freely? Aside being inside the blood vessels, what prevents them from clumping together when inside plain tube?
It is understandable that the blood clots once it is removed from the blood vessels and this clot can only be prevented by adding anticoagulant (a chemical that chelates calcium responsible for initiating blood coagulation and clotting). There is definitely something that keeps red blood cells from flocculating and this particular mechanism is what we intend to unravel. Let's stuff the structure of the blood cells, hopefully, it will help a lot with the explanation.
The red blood cell structure and the origin of their surface charge
The red blood cell is naturally made up of proteins and lipid bilayer that confers structural integrity to it. These molecules are responsible for the longevity and movement of the red blood cells throughout their life span of 90 days in the body's circulatory system.
The red cell membrane skeleton is a pseudohexagonal meshwork of spectrin, actin, protein 4.1R, ankyrin, and actin-associated proteins that laminate the inner membrane surface and attaches to the overlying lipid bilayer via band 3–containing multiprotein complexes at the ankyrin- and actin-binding ends of spectrin. The membrane skeleton strengthens the lipid bilayer and endows the membrane with the durability and flexibility to survive in the circulation.
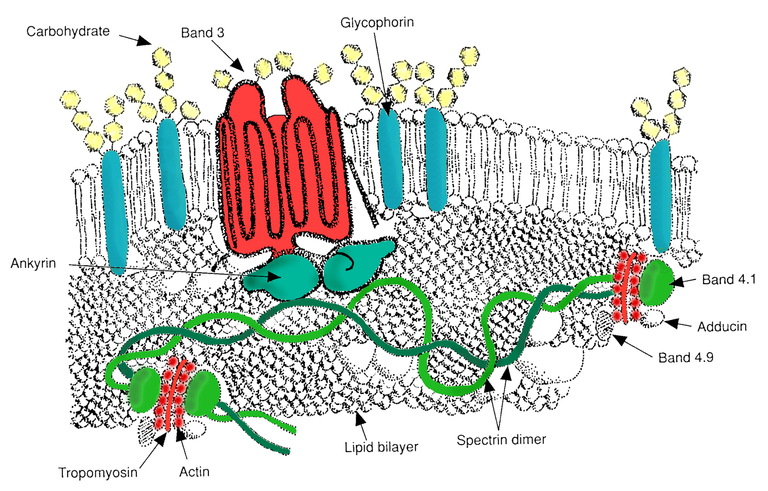
RBC membrane major proteins
The blood cells are naturally negatively charged dues to the components of their structural membrane. The carboxyl group of the N-acetyl neuraminic acid (NANA) aka sialic acid is the molecule that confers this negative charge. By virtue of this, red blood cells will always attract positive charges.
Ionization of the carboxylic group causes the blood cells to gain negative charge on its surface. if you recall from the elementary laws of electrostatic charges, like charges will always repel each other while unlike charges will attract.
The sum effects of the repulsive charges created by the carboxyl groups of sialic acids is known as the electrical Zeta potential. This charge is not only associated with red blood cells, but it has been observed to be commonly noticed in particles suspended in a fluid.
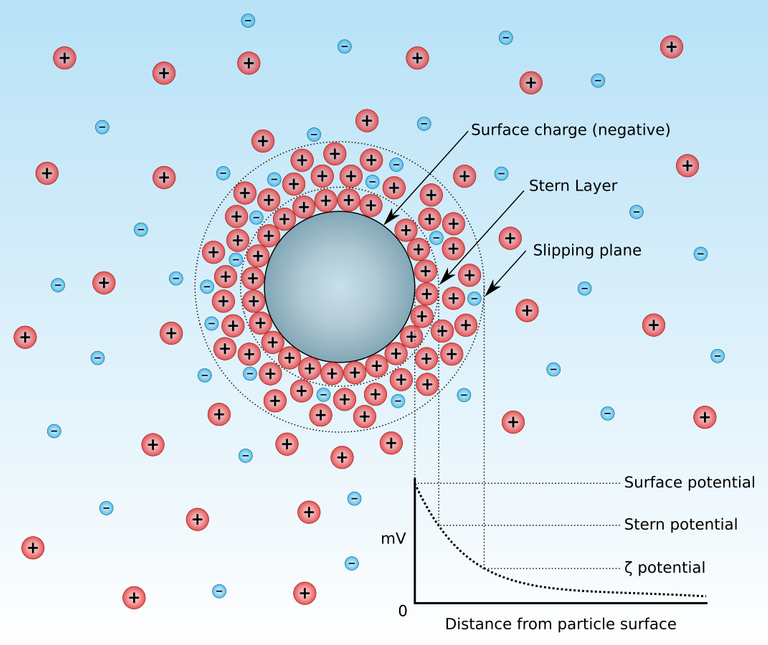
Zeta potential
The zeta potential is a physical property which is exhibited by all particles in suspension. The development of a net charge on any particle affects the distribution of ions in the surrounding interfacial region resulting in an increased concentration of counter ions, ions of opposite charge to that of the particle, close to the surface. Thus a double electrical layer exists around each particle.
These charges are responsible for the repulsive nature of red blood cells against one another. These charges ensure that the red blood cells no matter how many they are, will always move solely and freely within the blood vessels. Though these charge may be very important to ensure non flocculation, they still have some disadvantages when it comes to diagnosis testing.
Recall our previous discussion on Coomb's testing. In this diagnostic procedures, zeta potential charges can be problematic because they could prevent red blood cell agglutination. This is why the addition of Bovine albumin in the stage two of cross matching is very important.
Bovine albumin is protein gotten from animal and primarily has the potential of breaking and reducing the zeta potential on red blood cell surfaces, so that agglutination reaction can occur. It does not only reduce the zeta potential electrical charges, but also reduce the dielectric constant (the capacity of charge storage) of the red blood cells.
In a nutshell, without zeta potential repulsive charges, the cells can aggregate and become flocculated. In this kind of situation which is obviously not normal, it can all point to a pathological condition. Let's briefly take a look at those disease condition that are could result to blood aggregation.
Disease conditions that could alter blood zeta potential
In red blood cells, the membrane proteins besides the carboxyl groups of the sialic acid contribute to the zeta potential of red blood cells. What these means is that, alterations in the structure of the membrane proteins can have a huge impact on the structural integrity of the red blood cell surface membrane. Remember, the surface membrane confers the rigidity and flexibility of the red blood cells.
Membrane proteins contribute to the total net electrical charge of cell surfaces and can alter ZP through variation in their copy number and changes in their intermolecular interactions.
Genetic conditions like Hereditary spherocytosis (a genetic disease condition that is caused by a mutation in the gene that codes for the membrane proteins - Spectrin (alpha and beta), Ankyrin, Band-3 Protein and Protein - 4.2, which all make up the surface membrane of the red blood cells.

RBC membrane major proteins
Hereditary spherocytosis usually results to the red blood cells having spherical shape. Cells with this kind of genetic abnormality are usually fragile and easily burst when placed in varying concentration of salts of different ionic strength. Because they are easily fragile, they get destroyed easily and this can culminate to severe anaemia in the patient suffering from this kind of disease condition.
One of the most important diagnostic testing procedures that can be used to detect this type of disease is known as the Osmotic fragility test. This test measures how well a red blood cell can withstand or prevent haemolysis while being subjected to different salts of varying concentration. We will discuss this test procedure later in our medical workshop episode, keep in touch.
Aside the genetic disease above, one interesting thing that research has been able to find out, is the fact that, infection with malaria of the species Plasmodium falciparum, has been discovered to alter the zeta potential on the red blood cell surface. In a research conducted by Fuyuki Tokumasu and colleagues in 2012, they were able observe a significant reduction in the zeta potential of red blood cells.
The normal mean value of the blood surface zeta potential according to report is -17.7mV. The research carried out by the above researchers showed that, when red blood cells are infected, the value drops to -14.6mV and when they used neuraminidase to remove the principal component that confers the zeta potential, the value drooped drastically to -6.06 in normal red blood cells and - 4.64, hence, clearly validating the fact that the carboxyl groups of sialic acid contributes the zeta potential charge.
One commonly noticeable feature of red blood cells that have been infected with plasmodium parasite, is the presence of tiny knobs on the cell surface. These knobs makes the blood surface rough and susceptible to adhesion to the blood endothelial surface. This rough surface also contributes to the hypercoagulability of the blood cells.
This possibly is the reason why red blood cells infected with plasmodium parasite are always prone to adhering to the wall of the blood vessels and this could ultimately results to vascular occlusion of the blood vessels by these infected red blood cells.
These factors can all together reduce the repulsive charges that prevent the blood cells from aggregating in-vivo. Aggregation of blood cells has severe consequences and can even prove fatal if not managed. They can block major blood vessels that carry oxygen and nutrients to the brain and other major organs.
The resultant effect of this would be tissue necrosis and other associated effects linked to tissue deprivation of nutrients. Fibrinogen, which is a very important protein that must be converted to fibrin during clot formation has been shown to interact with the sialic acid in red blood cells.
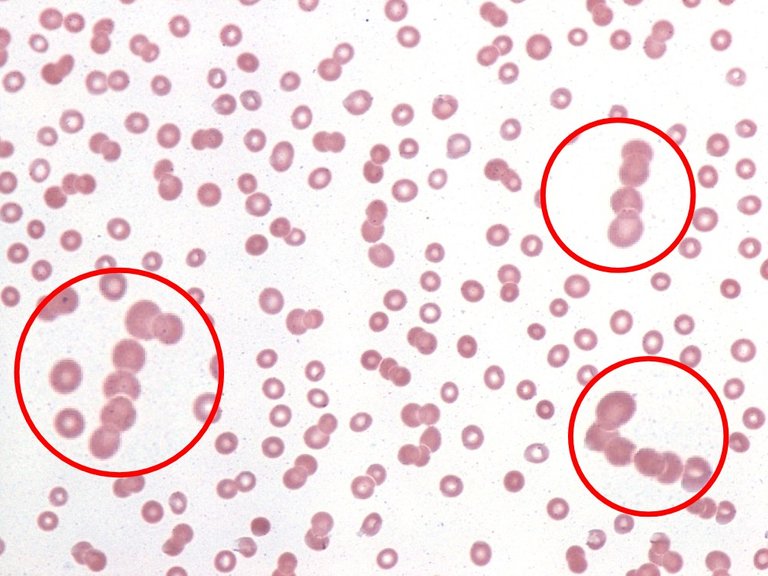
Rouleax formation in a peripheral blood smear from a patient with plasma cell myeloma
By implication, this interaction contributes to the aggregation of res blood cells so as to arrest bleeding especially in cases of tissue injury. Furthermore, when plasma proteins are excessively raised in conditions like multiple myeloma. This condition reduce the sialic acid content of the blood cells, hence, causing what is known as rouleaux formation in blood cells.
In rouleaux formation, the red blood cells as seen as packs of cells that lay on one another, hence forming a kind of long chains of red blood cells. Commonly, you see rouleaux formation of blood in sickle cell patient's samples. This condition contributes to the increased Erythrocyte sedimentation rate (ESR) gotten when analysis is done with their sample.
In conclusion, all of the above simply shows how important zeta potential is for red blood cells and to us as humans. Blood flocculation is bound to occur if the integrity of the structural membrane of red blood cells become questionable. All things being equal, our blood will continue to repel each other for our safety.
Until I come your way again, stay awesome!
References
•Bovine albumin function
•In-depth expository into Coronavirus Vaccine-Induced Thrombotic thrombocytopenia and associated side effects
•Anatomy of the red cell membrane skeleton: unanswered questions
•Electrical properties of the red blood cell membrane and immunohematological investigation
•Modifications in Erythrocyte Membrane Zeta Potential by Plasmodium falciparum Infection
•Sialic acid in fibrinogen: effects of sialic acid on fibrinogen-fibrin conversion by thrombin and properties of asialofibrin clot