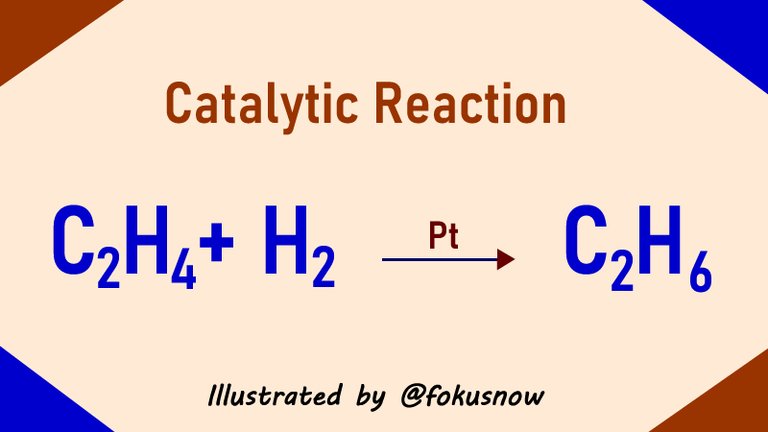

Introduction
In the last article, I wrote about different forms of chemical reactions. We looked at 3 types specifically - reversible reactions, decomposition, and combination reactions. Today, we will look at one important form of chemical reaction known as catalysis. We will take a little detailed look at it and how it works. First lets understand what catalysis is.

What is Catalysis?
Certain substances can affect how fast a chemical reaction happens. Some substances increase the speed while others reduces the speed. Such substances that change the rate of a chemical reaction are known as catalyst. While they do affect chemical reactions in terms of speed, catalyst still retain their chemical properties at the end of the reaction. They also maintain their quantity too.
A chemical reaction in which catalysts are used to speed up or reduce the rate of reaction is known as catalytic reaction. The process of itself of reducing or increasing the speed of a reaction is known as catalysis.

Types of catalytic reactions
There are majorly two types of catalytic reactions. They are as follows:
- Heterogeneous catalysis: In this type of catalysis, the catalyst exists in a state or phase different from the reactants. Usually, it exists in a solid state while the reactants exist in a different state like gaseous. Heterogeneous catalysis has so much value in industrial chemistry.
For example, in the industrial production of Ammonia from Nitrogen and Hydrogen, Iron is used as a catalyst for the reaction, helping to maximize the yield of ammonia from the the two reacting elements. Consider the reaction below:

Take note in the above reversible catalytic reaction that Ammonia was formed from Hydrogen and Nitrogen. The states of the reactants and products are different from that of the catalyst iron. Nitrogen, Hydrogen and the Ammonia all exist in gaseous state, while the catalyst iron exists in solid state, hence, it is a heterogeneous catalysis.
- Homogenous catalysis In homogenous catalysis, the reactants, products and catalyst exist in the same state. A very good example is the oxidation of sulphur (IV) oxide to sulphur trioxide. This is a catalytic reaction where nitrogen(II) oxide acts as a catalyst.
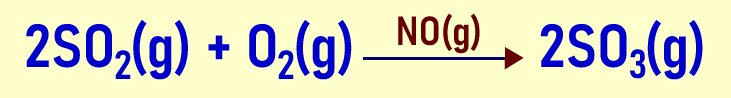

Nature of a catalyst
Catalysts maintain some chemical and physical properties that make them so unique in any chemical reaction. Consider the following:
1. Unchanged: Catalyst maintain the chemical characteristics and mass before, during and after the reaction. Because of the nature of reactants, catalyst might change in color and other physical properties, but they remain chemically unchanged.
2. Quantity: Catalyst always affect the rate of a chemical reaction no matter the quantity present. Usually, the more catalysts there are, the more change they make either to speed up or reduce the speed of a reaction.
3. When effective: Catalyst are only effective when used in an ongoing reaction. A catalyst cannot be used to start a chemical reaction because they are not active in such state. To improve the effect of a catalyst in solid state, its surface area need to be enlarged. The more a solid catalysts surface area, the more effective.
4. Function: A catalyst is there to either increase or reduce the rate of chemical reactions. It does not in anyway affect the type or nature of products gotten from the reaction. Because of this, the catalyst is written on top of the arrow when writing a chemical equation. A catalyst also does not also alter the chemical equilibrium of any reaction.

Types of Inorganic catalysts.
Inorganic catalysts are grouped largely into two based on whether they speed up a slow reaction or slow down a fast reaction.
- Positive catalyst: This type of catalyst speeds up a slow reaction. In industrial chemistry, they are very important. Some reactions are so slow they might take hours, days or even weeks to complete. For such reactions to happen at the industrial scale, positive catalysts are included. Check the example below:
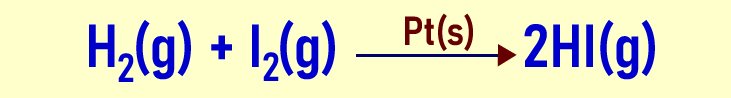
The catalytic reaction above show the formation of hydrogen iodide, a hydrogenation reaction too. The catalyst used is platinum. It helps to speed up the reaction, making it possible to happen at industrial scale.
- Negative catalyst: Negative catalysts slow than a reaction that would otherwise happen at a very fast rate. Consider the example below.


Conclusion
Catalytic reactions are very important in industrial chemistry. From example in the production of fertilizers, Nitrogen and Hydrogen are used to produce ammonia in large quantities. This is an example of a catalytic reaction and it used very useful at scale. Other forms of catalytic reactions remain so valuable to scientists in the field. In our next presentation, we will see other forms of chemical reactions.
