Magma chambers formed by hot areas in the earth's mantle lurk deep under the world's geysers and hot springs. The magma crystallizes as it comes closer to the earth's crust, forming new hot igneous rocks. Groundwater is driven to the surface by the heat from these rocks, and as the water cools, ions precipitate as mineral crystals. Quartz crystals made of silicon and oxygen, feldspar crystals made of potassium, aluminum, silicon, and oxygen, and galena crystals made of lead and sulfur are just a few examples.
A fascinating aspect is that many of these crystals have distinctive shapes, such as a cascade of pointed quartz or a stack of galena cubes.

Pointed Quartz crystal cluster; Author: JJ Harrison ; License

Galena with some golden colored pyrite from Huanzala mine, Huallanca, Bolognesi, Ancash, Peru ; Author- Ivar Leidus ; License
What drives them to grow into these shapes over and over again remains a mystery. The atoms provide an explanation for this feature of crystals. Every crystal's atoms are grouped in a highly structured repeating pattern, which is the crystal's defining property.
Sugar, ice, chocolate, ceramics, metals, DNA, and even some liquids are all known to have crystalline formations. The atomic arrangement of each crystalline substance belongs to one of six families: cubic, tetragonal, orthorhombic, monoclinic, triclinic, and hexagonal. Crystals will grow into geometric shapes that reflect the groupings of their atoms under the correct conditions.
Galena, for example, has a cubic structure made up of lead and sulfur atoms. The comparatively massive lead atoms are organized in a 3D grid at 90 degrees from one another, while the relatively small sulfur atoms are sandwiched in between.
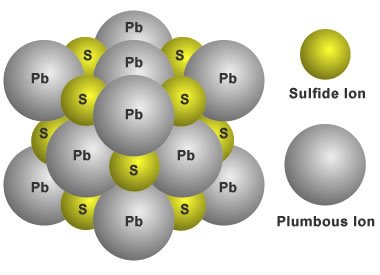
Structure of galena ; Source
Locations on the surface of lead attract sulfur atoms as the crystal grows. Lead will tend to keep bonding to the attracted sulfur atoms until the grid of bound atoms is complete. This means that the apparent shape of the crystal reflects the Galena crystalline structure's 90-degree grid arrangement.
Quartz, on the other hand, has a hexagonal crystalline structure, which implies that its atoms are arranged in hexagons in one plane.
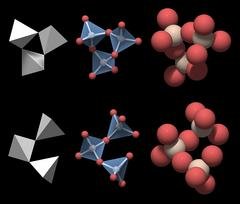
Basic structural unit of quartz ; Source
In 3D, these hexagons are built up of several overlapping pyramids, each made up of one silicon atom and four oxygen atoms, resulting in a six-sided column with pointy tips, which is the distinctive shape of a quartz crystal.
Most crystals have the ability to generate a variety of geometric shapes depending on their surroundings. Diamonds, for example, develop deep inside the earth's mantle and have a cubic crystalline structure that can grow into cubes or octahedrons! The shape of a diamond is determined by the conditions in which it is formed, such as pressure, temperature, and chemical environment. Because scientists can't see the mantle's growth conditions up close, laboratory research have proven that diamonds grow into cubes at lower temperatures and octahedrons at higher temperatures. The shape of a diamond can also be influenced by trace concentrations of water, silicon, germanium, or magnesium.
It's a good thing we know that diamonds don't grow into the shapes we see in jewelry. Those used in jewelry have been trimmed to show off their luster and clarity. Environmental factors can also influence whether or not crystals form, like in the instance of glass, which is produced of melted quartz sand but is not crystalline. Because glass cools quickly, the atoms don't have enough time to arrange themselves into the ordered structure of a quartz crystal. Instead, as the heated glass cools, the atoms' random arrangement is preserved.
Because they develop in such close quarters with other crystals, many crystals do not create geometric shapes. Although some rocks, such as granite, have visible crystals, none of them have recognizable shapes.
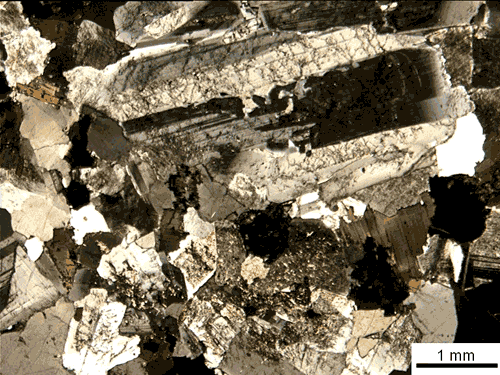
Thin section of granite ; Source ; License
Many minerals within magma crystallize at the same time as it cools and solidifies, soon running out of space, while many crystals, such as turquoise, don't form into any apparent geometric pattern in most environmental conditions, even when there is enough space.
While the atomic structure of each crystal has its own set of features that have nothing to do with human emotional requirements, they do have a lot of uses in materials science and health. In my postgraduate dissertation research, I recently used the crystallization process. I'll be posting a blog and updates on my research progress here soon.
You guys are incredible for reading to the end, and I hope I've helped to open our eyes to these lovely blossoms. Remember the wonderful science that went into the creation of these items the next time you add sugar to your tea or use ice cubes to cold your fizzy drink on a hot day! Friends, cheers!
Check these out for more content:
Crystallization of Magma.)
Quartz
Galena: The primary ore of lead that is sometimes mined for its silver content
Magma
The Chemistry and Structure of Diamonds
Posted from HypeTurf

