
Pixabay
The concept of electrolysis and how it affects silver is explained below. Electrolysis is an electrochemical process that uses electricity to cause indirect chemical reactions. The process takes place in an electrolytic cell with two electrodes immersed in an electrolytic solution:
Anode - the right electrode where oxidation occurs.
Cathode the negative electrode where the reduction reaction takes place.
Electrolysis of Silver
Pure silver can be purified by the process of electrolysis. In this process, impure silver is used as the anode and pure silver alcohol is used as the cathode. The electrolyte is an aqueous solution of silver nitrate (AgNO3).
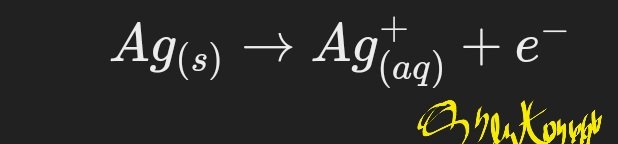
Process:
1. When an electric current is applied, silver atoms at the anode are oxidized, forming silver ions (Ag^+) and releasing electrons:
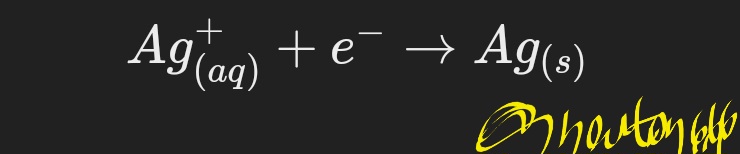
2. These silver ions pass from the electrolyte to the cathode, where they are recovered and stored as metallic silver:
3. The non-ionizing substances in solution remain at the anode as anode sludge.
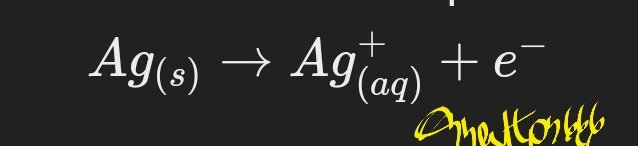
Now we have something unique: this process produces very pure silver at the cathode. Electrodeposition of Silver Electrolysis also uses a silver coating, a process called electroplating or electrodeposition. Use an element (can be any conductor) as the cathode and silver as the anode.
The electrolyte is another silver nitrate solution.
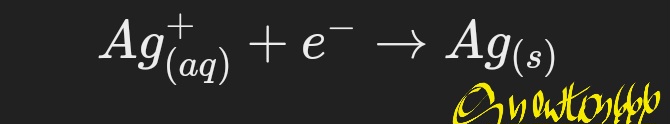
Process:
1. At the anode, silver atoms are oxidized to silver ions:
2. The silver ions move to the cathode, where they return and are deposited on the material:
3. Over time, the silvery-yellow layer separates. material. cathode.
Something Applications: This method is widely used in the production of jewelry, utensils, electrical contacts and other items that require silver plating or decoration. Electrochemical reactions The main electrochemical reactions in a silver electrolytic cell are:
Anode (oxidation):
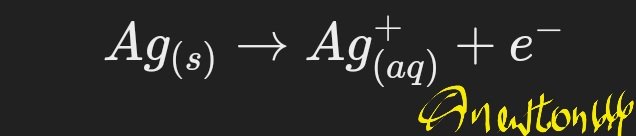
Cathode (reduction):
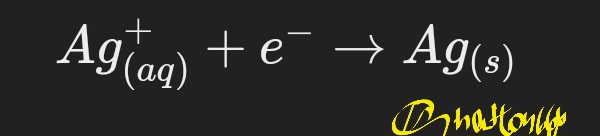
Current and voltage: The performance of the system depends on the current input and the voltage. The correct voltage is only necessary to prevent further reactions so that the silver melts and remains. Cleaning the electrolyte: Impurities present in the electrolyte can affect the quality of the coating and the performance of the system.
Electrolysis is an important process in the handling of silver due to its purity and use in metal plating. This electrochemical process allows the use of pure silver at an atomic level, resulting in high purity and quality products for industry and cosmetics.
Bibliographic Reference.
Electrochemical Engineering by C. L. Mantell, 2021.
General Physics by Santiago Burbano de Ercilla, 2003.
