We drink water a lot, and when we talk about hydration, a lot of us we mention that it has to do with our body having a lot of water which is a very good response for the word "hydration" but then some people would define it in a more distinct way and hopefully, we get there in this post.
If you go through the internet and you search for the water in humans, you would see words like we are made up of 60% water, and while we can go for days without food, we cannot go for days without water as it can lead to death. These sources aren't wrong, in fact, they are calling our attention to the fact that water is vital, water is sustaining, water is indestructible, and dehydration can be very bad for us.
When you see signs like dry mouth and lips, dry eyes, dark urine, headache, cramps, fatigue, and confusion, before you pick anything else as a possible cause, ask if the person is not dehydrated because those are the signs of dehydration. When we drink water and we are trying not to be dehydrated, we need to also not overdose on water so we ensure we do balance.
Talking about balance, our body needs to balance between intracellular and extracellular water. If the body, we have the cell and the organs, and there are fluids inside the cells which make up about 40% of the body weight, and there are fluids outside the cell which is about 5% of body weight. In the body, water likes to combine with things just as it like to dissolve and combine with things outside the body. Water is the solvent that dissolves solutes giving solution.
So with my explanation above, I would say that hydration is the body reaching a solution state where solvent is balanced with solute (minerals) both for intracellular and extracellular known as cellular isotonicity. Water gets into various cells and mixes with minerals in the body via osmosis. Water goes to places in the body solute are in a high concentration in the body and in doing so, the body is hydrated properly.
So what about electrolyte, they also help me hydrated. Good! I will explain electrolyte so we can understand hydration well. You see, spring water gets its minerals from the soil, so it isn't like water doesn't have minerals in them. Also, every mineral is gotten from the periodic table and when we talk about electrolytes, we are mentioning minerals that influences fluid balance in the body. Wherever there are minerals that can be dissolved in water, water is always around. For instance in the intracellular space, minerals like Magnesium, Potassium, ad Phosphate are present there, while calcium, chloride, and sodium are in the extracellular space, and water follow them.
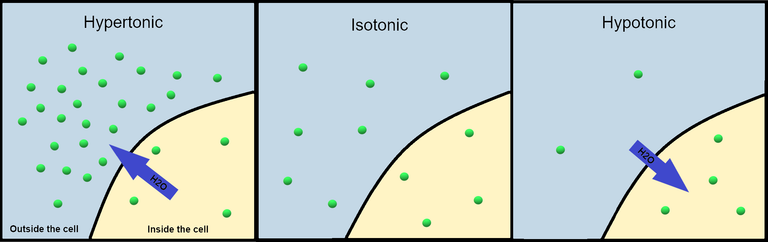
When we take Pwater, they go into our cells causing them to swell and this is because first, the cell membrane is permeable and since there are solutes in our cells that needs to be attended to, the water rushes into our cells and begin to dissolve them but then it should not burst it because just as water can get it, it can also get out as a result of the permeability of the cell membrane. But then, intracellular and extracellular osmolarity must be matched, else cells would be damaged.
Being overhydrated or underhydrated is bad and in the case of overhydration, it can lead to osmotic stress when the water is leaving the cell. When there is excess solute in the extracellular space especially sodium, it could lead to cellular crenation where water leaves the cells to the extracellular space which can lead t cell damage but this doesn't happen always as a result of Osmolytes which acts a intracellular solute that keeps water from leaving the cells. When cells that make up tissues do not have water in them then they shrink and this is when dehydration occurs as there is no solvent to mix the increased solute concentration.
Reference
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3438915/
https://pubmed.ncbi.nlm.nih.gov/24715560/
https://www.usgs.gov/special-topics/water-science-school/science/water-you-water-and-human-body
https://www.nhs.uk/conditions/dehydration/
https://courses.lumenlearning.com/suny-ap2/chapter/water-balance/
https://bio.libretexts.org/Learning_Objects/Worksheets/Biology_Tutorials/Diffusion_and_Osmosis
https://opentextbc.ca/biology/chapter/22-1-osmoregulation-and-osmotic-balance/
https://unacademy.com/content/neet-ug/study-material/biology/what-is-crenation-anatomy/
https://study.com/academy/lesson/intracellular-fluid-definition-composition.html