Coagulation:
The goal of coagulation is to make the colloidal system unstable by introducing chemicals that may eliminate the forces that are present on the surface of colloidal particles suspended in water in order to neutralise the negatively charged particles and allow them to agglomerate into flocs. Such chemicals are referred to as coagulants.
Ferric chloride (FeCl3) is one of the coagulants that is widely utilised. When 162 mg of ferric chloride (FeCl3) dissolve in one litre of water, 55.8 mg/L of ferric ions (Fe3+) and 106.5 mg/L of chloride ions (Cl-) are formed as a result of the easy dissolution of ferric chloride in water.

Anhydrous iron(III) chloride
The coagulation process are impacted by pH, as the pH value lowers when dosages of iron chloride are added because the creation of iron hydroxide flocculants removes the hydroxide ions.
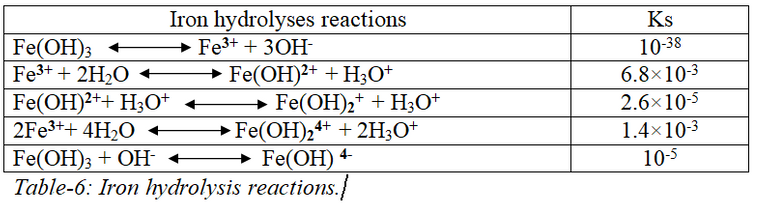
[Made using "MS word" and "Paint"]
- Electrocoagulation:
Trivalent ions are supplied at a concentration of 0.025 mol/m3, and electrostatic coagulation is utilised to treat the wastewater because of the high concentrations involved. Removable flocculants are created when the colloid's outer particles clash and become destabilised. The positive ions' attraction to the negatively charged colloids is the cause of this.
Rapid mixing is necessary after adding coagulant dosages to the water because inefficient processing might result from sluggish mixing.
Agglomeration:
Agglomeration is a process that aggregates tiny bodies suspended in aqueous medium into bigger blocks to hasten the settling process. Brownian motion causes the colloidal particles to first form particles with dimensions of about 0.1 micron after they have been released from their charge as a result of coagulation. These particles then begin to gather in larger groups under the influence of external mechanical movement (quiet moving), or of adhesion with coagulants.
References:
- [Introduction to Water Chemistry (Pollution- Treatment- Analysis). Dr. Nasser Al-Hayek. Publication of the Higher Institute for Applied Sciences and Technology (HIAST). Syrian Arab Republic, 2017.]
- Taparhudee, Wara (2002). "Applications of Paddle Wheel Aerators and Diffused-Air System in Closed Cycle Shrimp Farm System" (PDF). Witthayasan Kasetsart (Sakha Witthayasat). 36: 408–419. Retrieved 26 April 2020.
- Unsafe water kills more people than war, Ban says on World Day". UN News. 22 March 2010. Retrieved 10 May 2018
- Raymond Desjardins- Livre: Le traitement des eaux- 2éme edition- Ecole Polytechnique de Montréal- 1997- ISBN 2-553-00643-8
- Drinking Water Treatment- EDX- Delft University of Technology.
- Book- Drinking Water: Principles and Practices- by Hans J C Van Dijk (Author), Jasper Q J C Verberk (Author), Peter J De Moel
