There are two main groups for colloids in water:
1- Hydrophilic colloids:
Hydrophobic colloids in natural water form hydrogen bonds with the water molecules. These colloids are composed of big molecules with an organic origin. In contrast to hydrophobicity, which is irreversible, these large molecules scatter and dissolve in aqueous conditions as a result of the processes that associate them with water, and this dispersion process is reversible, so they may be put back together. Proteins, double sugars, humic substances, soap, and lignin are a few of the most significant hydrophilic colloids.
2- Hydrophobic colloids:
Given that soil is the cause of the turbidity of natural surface waters, silica particles, many colloidal deposits, and to a lesser extent soil are among the most significant hydrophobic colloids.
- When colloidal particles are exposed to an electric field and their motion is observed, electromigration (a phenomena where some of the particles travel in one direction, towards the negative pole, while others move in the other direction) is observed.
- Despite the fact that positively charged particles can occasionally be observed, most colloidal particles in natural water have a negative charge. Additionally, the colloidal particles pick up their charge either by ionization the component particles or by selectively adsorbing ions from the aqueous media in which they are present.
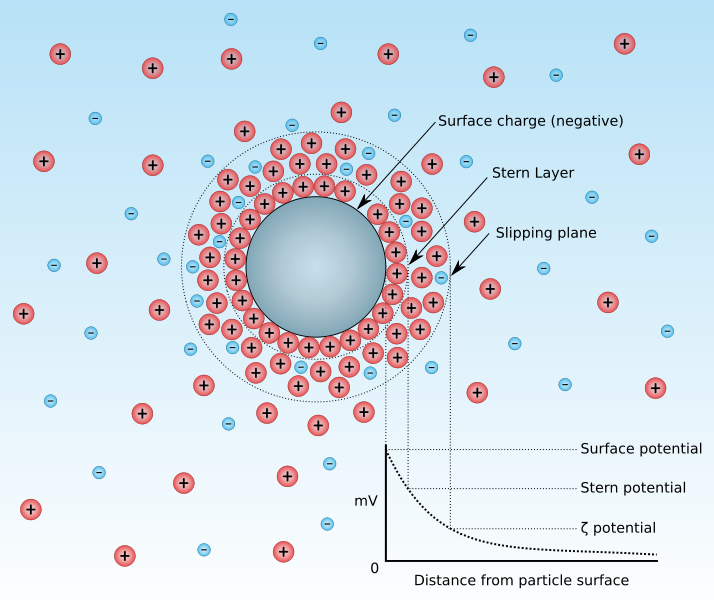
Micelle:
The existence of two fundamental layers (bilayer) in these particles has been proven by various studies. Thus, the dispersed phase material and dispersion medium (solution) are therefore in a clear physico-chemical connection, and the colloidal element forms a complex that is sometimes bound by a solid surround of solvent molecules and at other times by an electrical bilayer of electrolytes. The term "micelle" refers to this collection of many elements that are connected (Figure-2).

Figure-2: Scheme of a micelle formed by phospholipids in an aqueous solution.
In addition to being a polar molecule, water always includes electrolytes formed by the ionisation of some substances dissolved in it. In the event that charged colloidal particles are present in this medium, they engage in an electric attraction process with the ions that have the opposite charges, and simultaneously push the corresponding charges away from the micelle and move the polar water molecules near them.
Figure-3: Diagram of steric stabilization and gel network stabilization in colloids.
The system as a whole, including the stable layer and the water molecules connected to it, migrates towards the opposite pole if an electric field is created in the medium. This rate of electrical migration, known as the potential gradient, is proportional to the potential difference between the two electrodes divided by the distance between them, as well is related to the potential between the diffuse layer's outer surface and the moving system's surface. Zeta potential or electrokinetic potential is the name of this potential (Figure-4).
The relation shown below determines Zeta potential:
Z= K.e.q / D
Z: Zeta potential.
e: Thickness of the diffuse layer.
q: The electric charge of the moving system.
D: The dielectric constant.
K: a parameter that depends on particle size, with values of 4π for big particles and 6π for small, spherical particles.
References:
- [Introduction to Water Chemistry (Pollution- Treatment- Analysis). Dr. Nasser Al-Hayek. Publication of the Higher Institute for Applied Sciences and Technology (HIAST). Syrian Arab Republic, 2017.]
- Taparhudee, Wara (2002). "Applications of Paddle Wheel Aerators and Diffused-Air System in Closed Cycle Shrimp Farm System" (PDF). Witthayasan Kasetsart (Sakha Witthayasat). 36: 408–419. Retrieved 26 April 2020.
- Unsafe water kills more people than war, Ban says on World Day". UN News. 22 March 2010. Retrieved 10 May 2018
- Raymond Desjardins- Livre: Le traitement des eaux- 2éme edition- Ecole Polytechnique de Montréal- 1997- ISBN 2-553-00643-8
- Drinking Water Treatment- EDX- Delft University of Technology.
- Book- Drinking Water: Principles and Practices- by Hans J C Van Dijk (Author), Jasper Q J C Verberk (Author), Peter J De Moel