The Zeta potential, or the rate of electrostatic migration, has an impact on how stable a suspended particle is. Zeta potentials for unstable suspensions are less than 20 millivolts, whereas those for stable suspensions are less than 50 millivolts.
The attraction forces (Van der Waals forces), as opposed to the electrostatic driving forces, promote the assembly of tiny particles.
In the field of water treatment, there are constantly new techniques to gather colloidal suspensions in big, quickly sedimenting masses by eliminating their stability (Figure-1). A abrupt drop in the zeta potential accomplishes this, allowing the particles to recombine in accordant with Brownian motion (Figure-2).
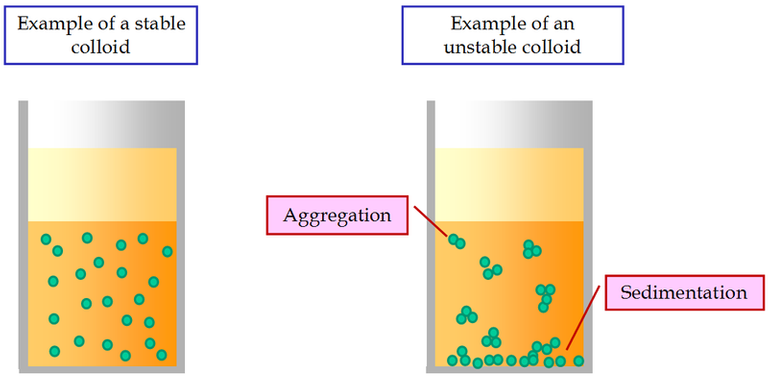
Figure-1: Examples of a stable and of an unstable colloidal suspension.
The concentration of suspended particles and agglomeration are connected. The sedimentation process moves quickly in concentrated solutions and becomes slower as the concentration drops.

Figure-2: Brownian motion of 350 nm polymer nanoparticles.
The action of eliminating the driving forces (Coulomb forces) and thus the occurrence of the process of suspended particle aggregation can be represented by the reduction of the zeta potential associated to the variance in potential of the interface between the fixed layer and the moving layer on the one hand, and the solution on the other.
The zeta potential is reduced by compressing the diffuse layer or extending the fixed layer, which lessens the influence of the suspended body's core charge and hence promotes instability. This role is often carried out by strong electrolytes, iron ions (Fe3+) and aluminium ions (Al3+) are the most significant of them, whereas its presence causes the diffuse layer's thickness to diminish and permits ions whose charges are opposite those of the centrosome to penetrate the fixed layer.
Numerous factors affect how agglomeration occurs, incorporating the medium's pH value, which influences the level of ionisation of the available salts, it affects the driving forces by causing ions to appear or disappear. Additionally, moving the medium increases the likelihood that the fine suspended particles will come into contact with one another, speeding up their agglomeration and sedimentation. Rapid stirring, however, might have the opposite result by breaking the produced blocks.
References:
- [Introduction to Water Chemistry (Pollution- Treatment- Analysis). Dr. Nasser Al-Hayek. Publication of the Higher Institute for Applied Sciences and Technology (HIAST). Syrian Arab Republic, 2017.]
- Taparhudee, Wara (2002). "Applications of Paddle Wheel Aerators and Diffused-Air System in Closed Cycle Shrimp Farm System" (PDF). Witthayasan Kasetsart (Sakha Witthayasat). 36: 408–419. Retrieved 26 April 2020.
- Unsafe water kills more people than war, Ban says on World Day". UN News. 22 March 2010. Retrieved 10 May 2018
- Raymond Desjardins- Livre: Le traitement des eaux- 2éme edition- Ecole Polytechnique de Montréal- 1997- ISBN 2-553-00643-8
- Drinking Water Treatment- EDX- Delft University of Technology.
- Book- Drinking Water: Principles and Practices- by Hans J C Van Dijk (Author), Jasper Q J C Verberk (Author), Peter J De Moel.
