If there is one precious gem with crebrities in recent times, it is Diamonds. Simple as its name might sound, it has been in existence for at least 2500BCE and scientists were on the quest to understand it for years. Diamond is so strong that it can only be scratched by another diamond, but what if I tell you that scientist are starting to replicate diamonds using we humans in the lab.
Diamond was first used when ancient chinese used it to polish stone tools and while we have grown to love this mineral, we have also learned that the chemica makeup of diamonds are similar to the chemical makeup of the lead in pencils. Actually scientists have learned that the formation of diamond is dependent on the temperature and pressure at which the carbon is formed. These two things are the determining factor to seeing if what you get is coal or diamonds.
Before the 20th century, all the diamonds we had on earth were made and gotten from the earth crust and while people had the carbon gem at hand, they didn’t even understand it even until as soon as the 18th century. It was in the late 1600s that scientists Giuseppe Averani and Cipriano Targioni decided to check if it could be destroyed. They used a lens that focused the sun ray on it thereby exposing it to high temperature. To their surprise, the diamond disappeared instead of breaking or melting. This same thing happened over again for every experiment that scientists tried.
When scientists decided to collect the gas from the vaporized diamonds into calcium and water solution, it became cloudy and this showed that the vapor contained carbon dioxide which showed that the compond that made up the prescious stone was carbon.
For scientist to be able to use humans to recreate diamonds, the carbon in dead humans needs to be removed first. While we are carbon based life, we are not entirely made up if carbon. Cremation helps to caporize other compounds leaving carbon behind in its pure form. 1 Cerat of Diamond is about 0.2 grams of total carbon.
The carbons are mixed with graphite and contained in a tiny diamond that will be the seed for crystalization when it is being heated under the right temperature and pressure. To understand the diamond process, think of the rock candy our of sugar process where a single crystal is put into a sugar solution causing the sugar crystal to stick to the crystal in the solution. In the case of carbon, it is heated and dissolved into molten metal.
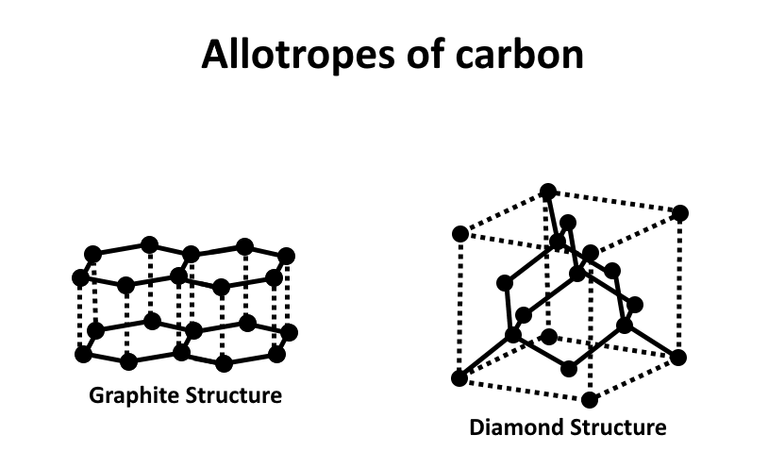
You wonder why the carbon is mixed with tiny diamond seed; it is because carbon can exist in different crystal forms including graphite,fullerene, graphene, and diamond. The form carbon takes depends on which of the crystal structure is most stable under the temperature and pressure. The difference between a synthetic diamond and a natural diamond is the ratio of carbon Isotope where organic carbon holds a higher ratio of carbon 14.
Reference
https://www.eterneva.com/resources/carbon-in-cremated-ashes
https://pubchem.ncbi.nlm.nih.gov/compound/Diamond
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Map%3A
https://www.diamondchemistry.com/pages/about
https://www.rsc.org/periodic-table/element/6/carbon#:~:text
https://pubchem.ncbi.nlm.nih.gov/compound/Diamond
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Map%3A
https://www.diamondchemistry.com/pages/about
https://www.rsc.org/periodic-table/element/6/carbon#:~:text