Reference Video Provided By Pierre Poilievre: https://www.facebook.com/PierrePoilievreMP/videos/1902256863313957
Messing with the slave masters pet monetary system can be a little dangerous. Ask John Kennedy, Executive Order 11110. They have used inflation and deflation to make 3rd world countries for a long time, used the printing press to make war possible, all sorts of dirty shit.
I have a couple of previously posted videos if you care to really zoom in on monetary system corruption:
https://peakd.com/hive-167922/@photonium/dcgoyexn
https://peakd.com/hive-167922/@photonium/zkkilvnp
Pretty cool he is covering Sudbury and Nickel. They say Sudbury was actually the site of a meteor landing, and that is how all that Nickel got to be where it is. Nickel is what they like to call, a siderophile, in that it likes to hang out with iron, or at least that was the case when the Earth was in a state of creation, becoming how it is the way we know it now. Or what they like to call, primordial Earth. So Iron, and all the elements close to it on the periodic table tend to be found deep in the Earth or the core.
Lithophiles are elements that liked to hang out with Silicon, and these guys tend to be found on the Earths surface.
Chalcophiles liked to hang out with Sulphur.
Atmophiles like to be gases and ended up being the Earths atmosphere.
So finding Nickel on the surface of the Earth, I don't think is that common. I've even covered some stuff that says if you find a rock of certain appearance that happens to have nickel in it, there is a good chance you have found a meteor.
Pulled this out of google: " The siderophile elements include the highly siderophilic ruthenium, rhodium, palladium, rhenium, osmium, iridium, platinum, and gold, the moderately siderophilic cobalt and nickel, in addition to the "disputed" elements mentioned earlier – some sources even include tungsten and silver."
Then I pulled this off of google: " Chalcophile elements, defined as those which have an affinity for a sulfide phase (e.g., Ni, Cu, Se, As, Cd, In), are essential components of many aspects of modern technology (e.g., photovoltaic cells, batteries, wind turbines)."
And now, I am left wondering, is Nickel a Chalcophile or is it a Siderophile? Maybe it's both?
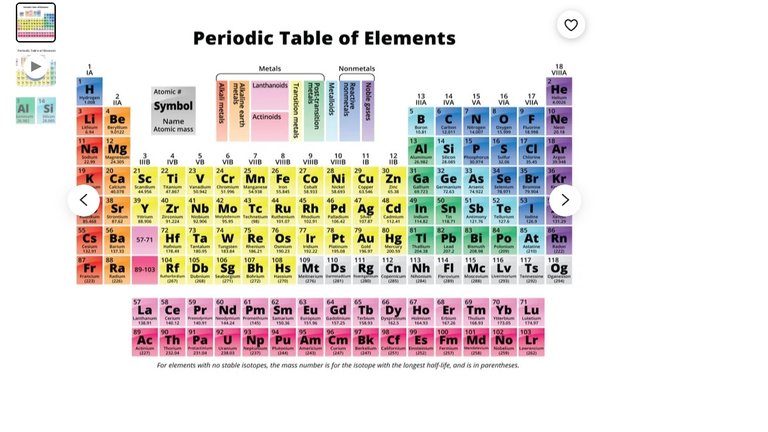
Going by the table, it sure looks like it is more close to Iron than it is to Sulphur.
I just pulled this off of google: Can an element be both siderophile and chalcophile?
"Most elements that are siderophile are usually also somewhat chalcophile and vice versa."
Ok, so I guess they can overlap, just so I can try to keep this story as accurate as I can.
Technically, nickel is typically used to galvanize steel, like those big cattle troughs, or roofing nails. Chromium is used to make stainless steel, like your kitchen sink or a DeLorean. In Pierre Poilievre video, he seems to be throwing around the word "stainless steal" in a somewhat casual way not conducive to it's exact definition. Not that, that is such a horrible thing.
When I did this post a month or two ago the price of Nickel which apparently they need in order to make EV batteries was at 19.93 USD per Kilogram. And it looks like that price has held it's position. Maybe they haven't updated the website since I last used it, or maybe the price is just really stable. The website does confirm the prices were updates as of 07-May,2022, so the price of Nickel is indeed quite stable, at least in terms of recent months.
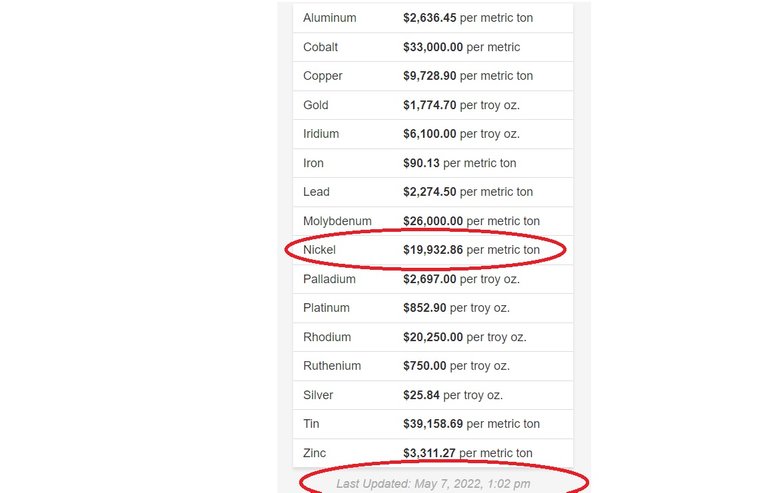
This is the website if you wish to check it out: https://www.metalary.com/
But, probably not a terrible idea to save any older Nickels you come across.
American Nickels:
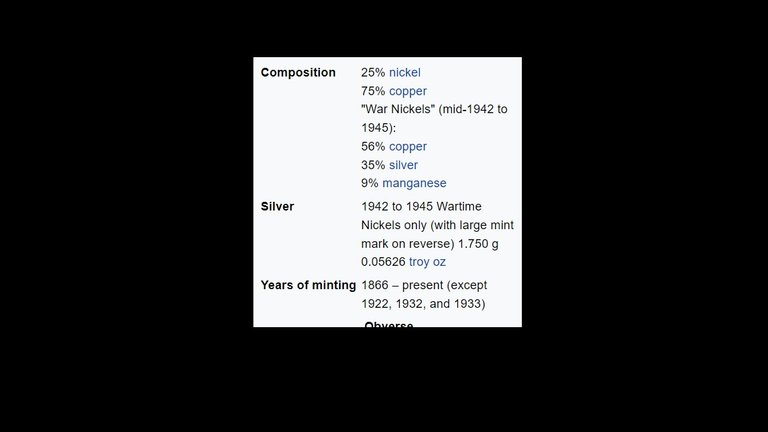
This is kind of the nutshell story of American Nickels
Canadian Nickels: Is basically covered already in the opening video by Pierre Poilievre. Most particularly by the following screen shot:

Far left Nickel coin: 2021
Middle Nickel coin: 2001
Far right Nickel coin:1981
Gist is, any Nickle 2021 or younger is pretty much crap.
Bonus video provided by You Tube Channel (Periodic Videos) -->
Thank you for reading, I like comments if you would like to add anything.




