Greetings to everyone! Take a moment to consider your hands. I insist, (haha) but please have a look, cause what we are about to learn is a common thing but has a very important part in chemistry. So, you checked your hands right? They look identical right? Normally 5 fingers on each hand with same finger sizes. OK, now try placing one hand on top of the other hand. and you'll notice an intriguing truth – they're mirror images, but they can't perfectly overlap. Much like this hand paradox, the molecular realm introduces us to a captivating concept known as chirality – a property that influences everything from the drugs we take to the very essence of life itself.
Let's picture, you're enjoying a refreshing cup of your favorite morning beverage. Whether you prefer a hot cup of coffee or a soothing mug of tea, chances are you've encountered chirality without even realizing it. The aromatic molecules that lend these beverages their distinct flavors are a perfect starting point to unravel the mysteries of chirality.
Chirality, derived from the Greek word "cheir," meaning hand, refers to the property of asymmetry in molecules that have a non-superimposable mirror image. The word is used for those objects which have right handed and left handed forms i.e., molecules which have handedness and the general property of handedness is termed chirality. An object which is not superimposed on its mirror image is chiral.
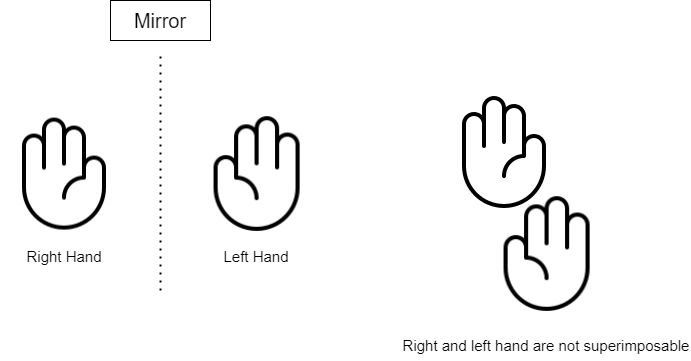
Enantiomers (optical isomers) occur only with those compounds whose molecules are chiral. Human hands are related to each other as object and its mirror image typical of enantiomers. Thus, one's right hand will fit into a right glove and not into a left glove. Now, it is true that chiral is the term that is used to describe molecules of enantiomers since these are related the same way that a right hand is related to a left hand. A structurally chiral system is the human body itself, the heart lies to left of center and the liver to the right.

Several plants display chirality by way they wind around supporting structures and thus represent as a left handed or right handed helix. Apart from one of the 20 amino acids, for the rest 19, the components of proteins are chiral and left handed. On the other hand, the molecules of almost all naturally occurring sugars are right handed. The enantiomers display physiological differences, for example; one enantiomeric form of a terpenoid called limonene smells like oranges while the other enantiomer smells like lemons. Interestingly, ones nose is capable of distinguishing between enantiomers, i.e., the receptor sites for the sense of smell are chiral.
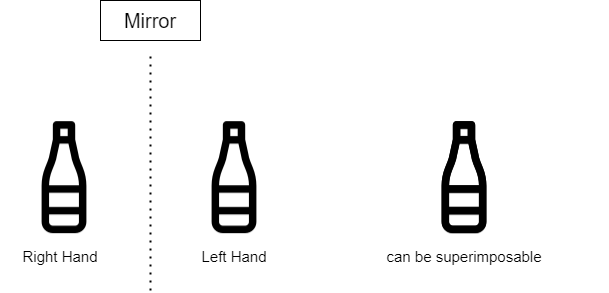
Objects and molecules which are superimposable on their mirror images, say for example a cup or a bottle is achiral. We can assume a hypothetical internal plane of symmetry which bisects an object or a molecule into mirror reflective halves. Such an object or a molecule with an internal plane of symmetry is achiral, basically the object can be superimposed on its mirror image. Thus, the bottle in the above diagram drawn by me is achiral since it can be divided into two equal halves by its plane of symmetry.
Although this topic is a vast topic in organic chemistry, with all those fancy and large molecules; I wanted to only bring to light the thing that these complex phenomena and complex chemistry is based upon real life concepts and can be applied on macro to micro levels. How interesting right! Chirality is truly a captivating aspect of the molecular world that influences everything from the structure of biological molecules to the efficacy of pharmaceuticals. I hope you liked the intricate dance between left-handed and right-handed molecules in this post.
Software used:
The diagrams are prepared using draw.io
I would like to invite you to join FreeCompliments community, a community created specifically for giving out free compliments! Be kind and share love :)

If you like my work and would like to support me, you can do so by joining my fanbase by clicking this link
Exploring Time-Independent Schrödinger's Wave Equation and Particle in a 1D Box | ChemFam #80 |
The Role of Gamma Function in Quantum Mechanics | ChemFam #79 |
Postulates of Quantum Mechanics and Normalization of Wavefunction |ChemFam #78|
Understanding Commutator Relations and Exploring Eigenfunctions in Quantum Mechanics |ChemFam #77|
How to find Expression of an Operator and Commutation Relations |ChemFam #76|
Basics to Quantum Chemistry: Operators, Functions and Properties of Operators |ChemFam #75|
A Comprehensive Study of Euler's Reciprocal Rule in Thermodynamics |ChemFam #73|
A Deep Dive into Nutrition Essentials: Your Path to a Healthier, Happier You |ChemFam #72|
Decoding Liver Function Tests through Chemistry |ChemFam #71|
Understanding the Dynamic Roles of Metalloenzymes and Metal-Activated Enzymes |ChemFam #70|
Cracking the Thermal Code: Differential Thermal Analysis in Modern Research |ChemFam #69|
Applications and Importance of IR Spectroscopy: Shedding Light on Molecular Structures |ChemFam #68|
The Silent Revolution: How Polymers are Shaping Our World? |ChemFam #67|
Beyond the Bin: The Many Faces of Plastic Management |ChemFam #66|
Spectrophotometry Simplified: The Beer-Lambert Law in Spectrophotometry |ChemFam #65|
Chromatography: Unraveling the Science of Separation |ChemFam #64|
Colorful Clues: The Magical World of Chemical Indicators |ChemFam #63|
Colloids in Action: Impacting Your Daily Life More Than You Think |ChemFam #62|
The Complex Landscape of Opioid Analgesics: Addressing The Concerns |ChemFam #61|
Genetic Engineering: Pioneering Progress or Ethical Predicament? |ChemFam #60|
The Guardians Against Microbial Menace: Antibacterial Agents |ChemFam #59|
The Cholesterol Conundrum: The Story of Statins |ChemFam #58|
PS The thumbnail image is being created by me using canva.com
Games I play on Hive
| Games I play on Hive | Game description |
|---|---|
| Terracore | Terracore is an Idle mining game based on Hive blockchain |
| Rise of the Pixels | ROTP is a Web3 browser game about game development on Hive |








