Howdy, fellas! When we gaze out the window or through our eyeglasses, or even through a set of binoculars or one of the several magnifying lenses available, one thing we do not notice is the glass itself. I'm sure many of us have pondered how something so solid could be considered "invisible."

Source; License
To understand what glass is, we must begin beneath the Earth's crust, where two of the most abundant elements, silicon and oxygen, react to generate silicon dioxide, whose molecules self-assemble into a regular crystalline structure called "quartz."
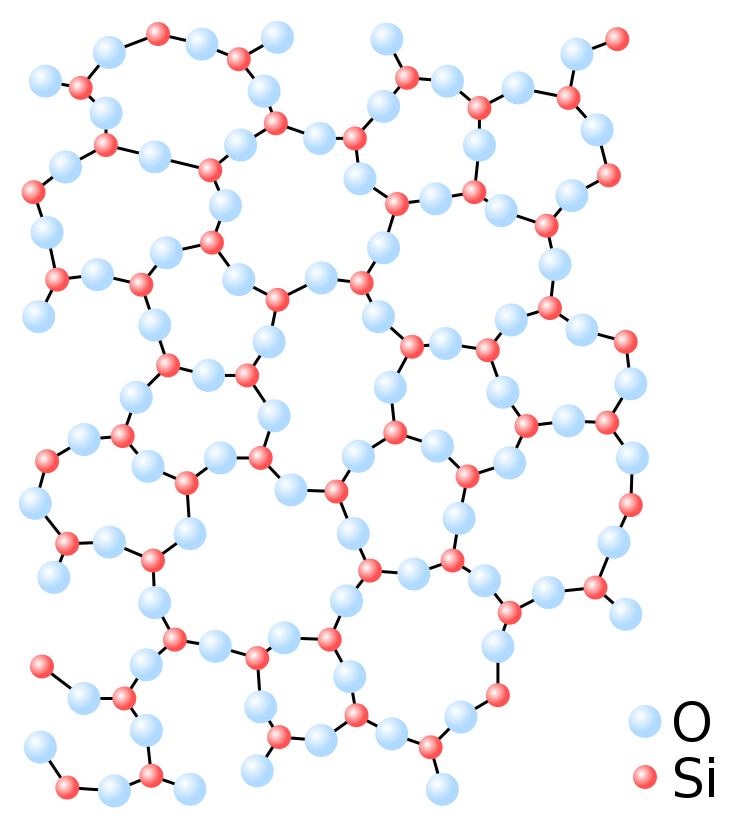
The amorphous structure of glassy silica (SiO2) in two dimensions.
Source ; License- Public Domain
Quartz is frequently found in sand, where it accounts for the majority of the material. Additionally, it is the primary component of the majority of glass kinds. We'd point out that glass does not appear to be made up of several tiny bits of quartz for a reason: the edges of the firmly formed grains and smaller imperfections within the crystal structure reflect and disperse light that attempts to flow through them.

Quartz sand (silica)- main raw material in commercial glass production
Source ; License
However, when quartz is sufficiently heated to a high temperature, the additional energy created causes the molecules to vibrate and break the bonds that hold them together, transforming them into a flowing liquid, just as ice melts into water. However, unlike water, liquid silicon dioxide does not crystallize when it cools. Rather than that, as the molecules lose energy, their ability to shift into an ordered position decreases, resulting in what is referred to as a "amorphous solid."
Amorphous solids have the chaotic structure of liquids, allowing molecules to fill in any gaps. This property uniformizes the surface of glass on a tiny level, allowing light to strike it without being scattered in all directions.
To understand why light can flow through glass, we need to dig a little deeper into the subatomic level of matter. It is well understood that an atom consists of a nucleus and electrons orbiting around it. It may surprise you to learn that it is essentially empty space, which is more than enough to let light to pass through without colliding with any of the atom's components.
The various energy levels at which electrons in an atom can exist significantly affect how light flows through the atom. Absorbing a photon of light that passes through an atom is sufficient to excite electrons and move them to a higher energy level, but the photon's energy must be just perfect; otherwise, the photon will pass by.
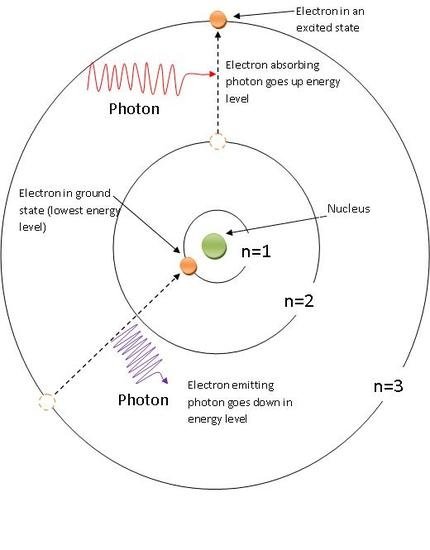
Source: ChemWiki
In glass, the energy level rows are too far apart for a photon of visible light to provide electrons with enough energy to jump between them. On the other hand, photons from ultraviolet light offer the exact amount of energy required and are absorbed, which is why we cannot get a suntan through glass.
On the other hand, photons from ultraviolet light offer the exact amount of energy required and are absorbed, which is why we cannot get a suntan through glass.
Glass's remarkable virtue of being both solid and transparent has enabled it to be used in a broad variety of applications throughout history.
It is utilized in windows to let light into our homes while keeping out the other elements of weather. Glass is utilized in lenses that enable us to see both the huge planets beyond our planet and the microscopic species that are ubiquitous on it.
Could a world without glass exist in contemporary civilization? Without a doubt, such a significant substance as glass is best known for its featurelessness and invisibility, and as a result, we appear to forget that the material exists when put in our line of sight.
Thanks for your time in reading, hivers!
For more details, check out these links:
Why Is Glass Transparent? Understanding Transparent Materials and Their Uses
Glass production
Glass
What Makes Glass Transparent?